The core technology of PuriBlood lies in the innovative patented technology – Surface Zwitterionization, it provides general types of materials with universal bio-inert compatibility for most biologically complex components such as human blood.
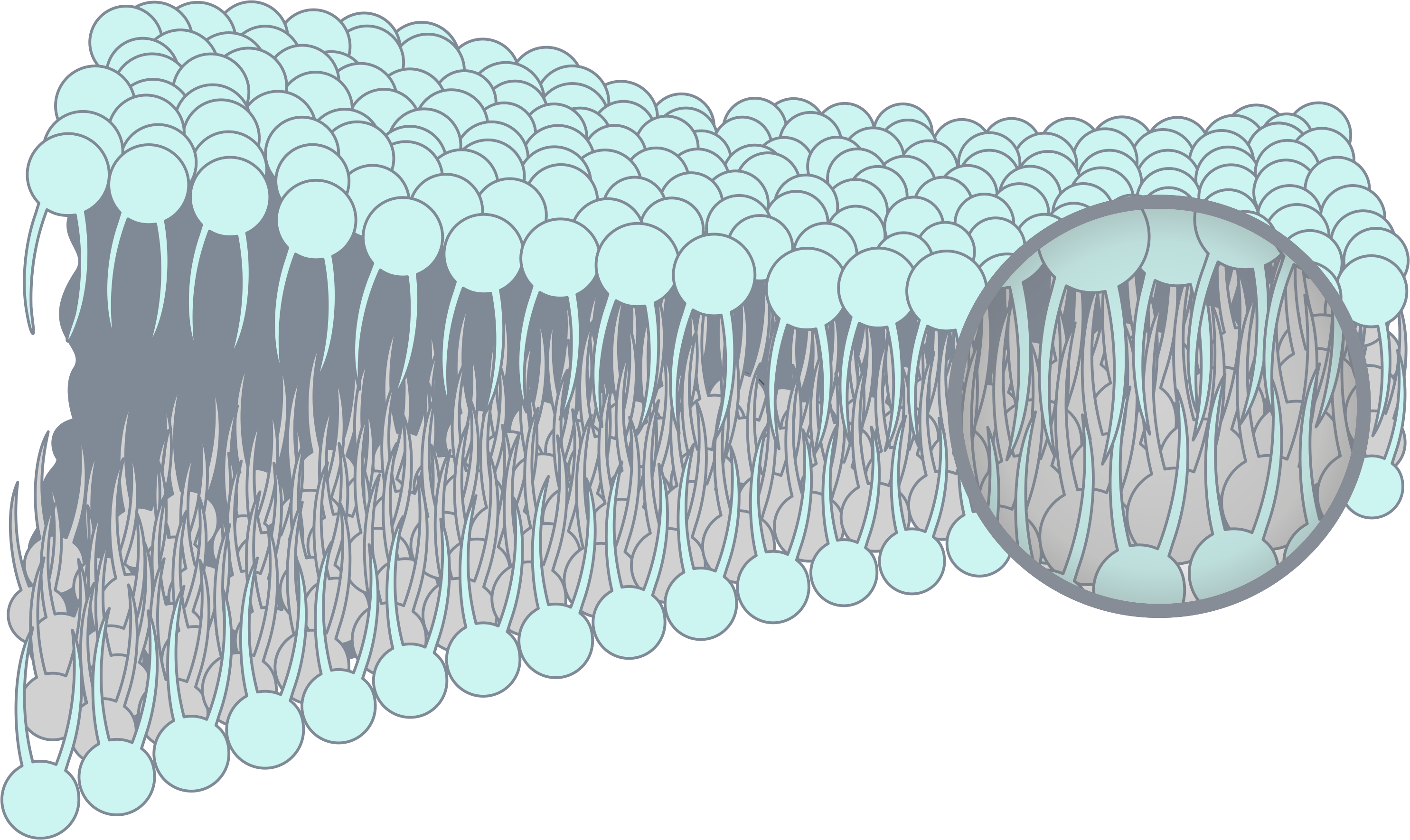
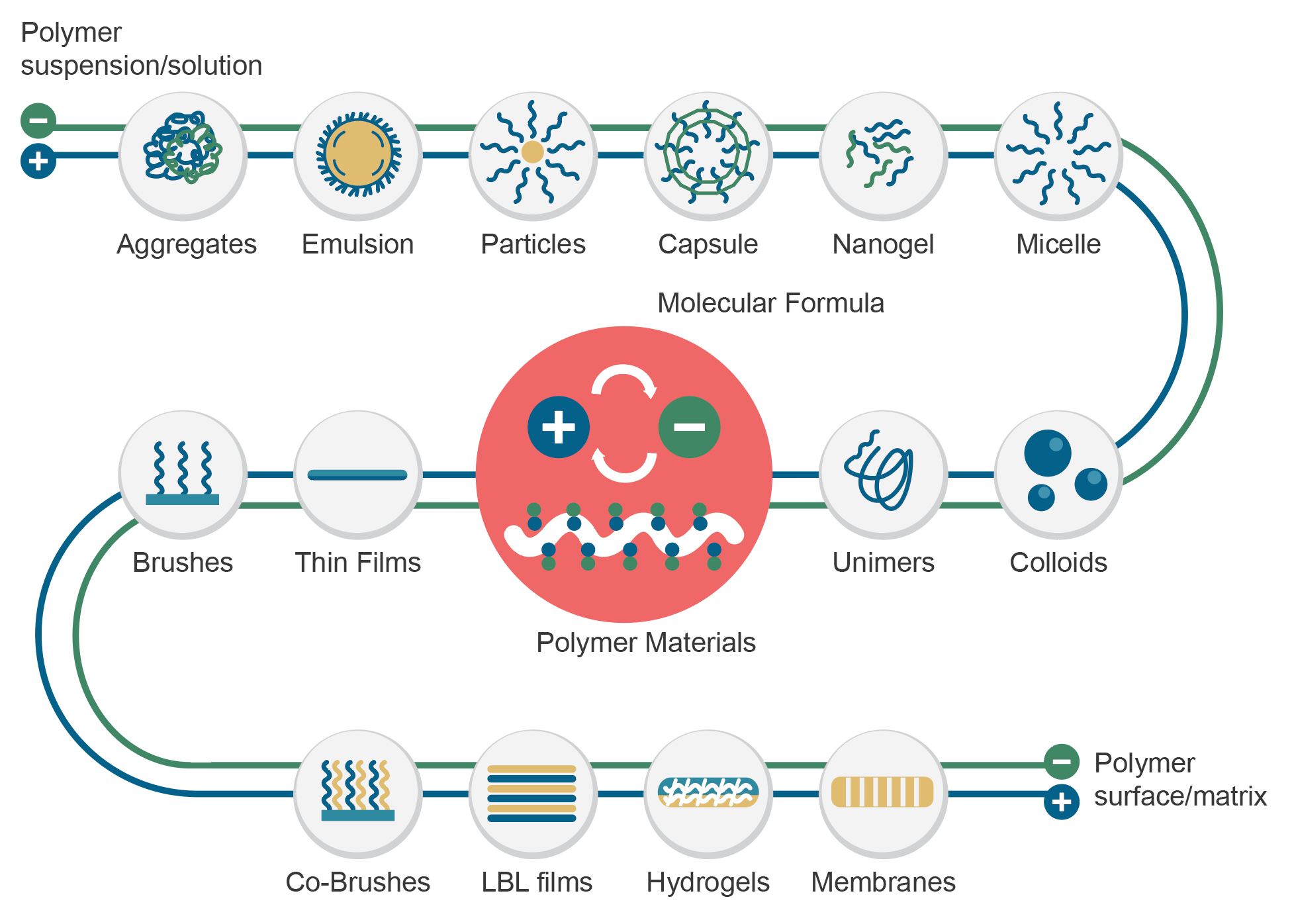
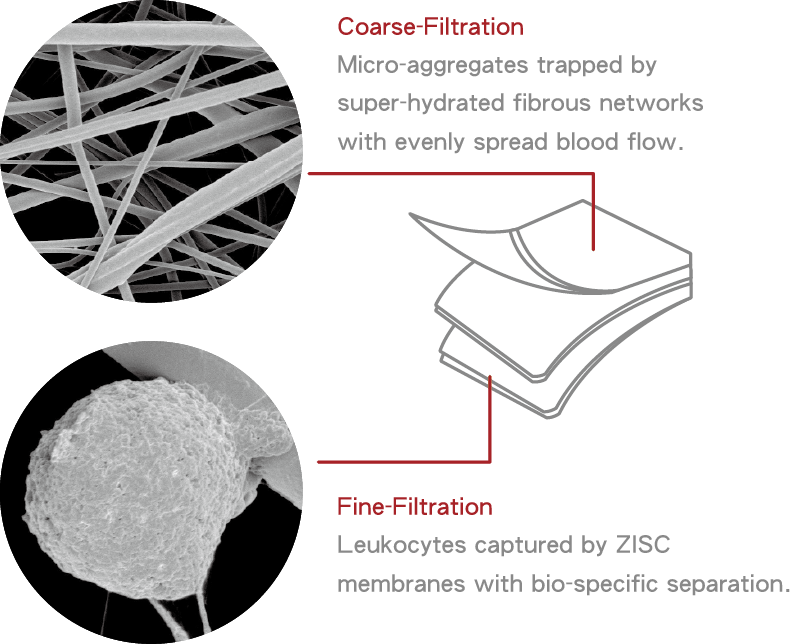
Our technology provides an advanced protection to support living beings – from medical devices to daily protection. It is characterized by its biocompatibility while high resistance to biofouling. Further through the design of the charge bias on the surface, it is capable of capturing specific cells for use with a variety of downstream research applications. – Allan Hoffman Zwitterionic technology (abbr. ZWI-TEC) was developed by Prof. Yung Chang and his research team at the R&D center for membrane technology (RDCMT) for a wider impact of biomedical applications from 2006. ZWI-TEC was inspired by the surface arrangement of the cell-lipid bilayer, which acts as a barrier in the cell, keeping proteins or biological substances from spreading to other places and preventing the entry of harmful substances. Similarly, zwitterionic materials creates a protective layer on the desired materials surface to prevent the contact with biological substances while maintaining strong hydrophilicity and high biocompatibility. The R&D team designed the charge bias on the zwitterionic surface (the so called Zwitterionic-Bias) on the basis of ZWI-TEC, making more advanced bio-applications possible. One of the studies was to capture stem cells from human blood. During this study in 2009, Prof. Chang discovered that white blood cells could be captured specifically on the zwitterionic-bias membrane surface. After 6 years of verification efforts, it was decided to lead the team to establish PuriBlood in 2016 and commercialize the research outcome as a leukocyte reduction filter. The broad characteristics of zwitterionic polymers include high hydrophilicity, low surface free energy, strong hydration, and weak biomolecule interactions, as a result, it produces adhesion resistance to common biological substances. With such a feature, this endows bioinert performance for the general types of polymer systems (such as PVC, PP, PE, or EVA). The design of zwitterionic composite materials can bring a new direction to the next generation of medical materials, devices, or equipment a safer and easier application. The Zwitterionic material could form into various polymer materials in following figures. Zwitterionic-bias technology is the NEW generation of bioinert and bioactive smart material, beyond HEMA, PEG and zwitterionic polymer systems. On the surface of the Zwitterionic membrane, we create a zwitterionic-bias neutral portion with a balance amount between positively and negatively charged moieties, which provide excellent inhibition in plasma protein adsorption, blood platelet adhesion and activation, and thrombus formation. On the top layers of membrane structure, Zwitterionic-bias technology creates a membrane that is highly hydrated and compatible with human whole blood which is designed to evenly distribute the blood flow and trap the clots and microaggregates. (coarse-filtration) In the core layers of membrane structure, Zwitterionic-bias technology provides a filter media with a specific affinity to selectively capture white blood cells and allows red blood cells to pass through the filter without rupture. (fine-filtration) Yan-Wen Chena, Antoine Venault, Jheng-Fong Jhong, Hsin-Tsung Ho, Chuan-Chuan Liu, Rong-Ho Lee, Ging-Ho Hsiue, Yung Chang, 2017 This study investigates the development of membranes for leukocytes depletion from model hydrogel interfaces. We first designed hydrogels from 2-(dimethylamino)ethyl methacrylate (DMAEMA) and poly(ethylene glycol) methyl ether methacrylate (PEGMA) to form poly(DMAEMA-co-PEGMA), and [2-(methacryloyloxy)ethyl] trimethylammonium chloride (TMA) and PEGMA to form poly(TMA-co-PEGMA). After characterizing the actual chemical composition of the hydrogels by XPS, efforts were made on studying the effect of the nature and content of amine functional group on the hydrophilicity and the surface charge of the gels. The adhesion of fibrinogen and blood cells was then methodically studied. Both types of gel are positively charged at pH 7.4, promoting fibrinogen and cell attachment. However, for the particular composition of 64% of DMAEMA in the hydrogel, results reveal that the adhesion of leukocytes was more important than that of erythrocytes and thrombocytes, thus suggesting that the control of the surface charge could lead to the selective adhesion of leukocytes. Based on these results, membranes were developed and tested in blood filtration for leukocyte depletion. Results prove that polypropylene membranes modified with a poly(DMAEMA-co-PEGMA) layer, containing 64% of DMAEMA can effectively lead to leukocyte depletion during blood filtration (99.6%) still maintaining a high erythrocytes recovery (98%). Cheng-Chi Lien, Po-Ju Chen, Antoine Venaulta, Shuo-Hsi Tang, Ying Fua, Gian Vincent Dizona, Pierre Aimar, Yung Chang, 2019 Although widely used in blood-contacting devices, polypropylene (PP) membranes are prone to biofouling by plasma proteins and blood cells. The present study explores the effect of a surface zwitterionization process on the improvement of the biofouling resistance of PP membranes for leukocyte reduction filters. The modification strategy consists in forming an interpenetrating network of poly(glycidyl methacrylate-co-sulfobetaine metha- crylate) (poly(GMA-co-SBMA) around the fibers of coated PP membranes, using a cross-linking agent: ethyle- nediamine (EDA). It is shown that with EDA, a range of poly(GMA-co-SBMA) concentration (1–5 mg/mL) leads to a 0°-water contact angle and high hydration of the networks without affecting the intrinsic porous structure of the material. Besides, the related membranes show excellent resistance to biofouling by Escherichia coli, fi- brinogen, leukocytes, erythrocytes, thrombocytes and cells from whole blood with reductions in adsorption of 97%, 86%, 90%, 95%, 97% and 91%, respectively, compared to unmodified PP. Used in whole blood filtration, it is demonstrated that in the best conditions (5 mg/mL copolymer, with EDA), leukocytes can be efficiently re- moved (> 99.99%) without altering the erythrocytes concentration in the permeate, and that leukodepletion is more efficient than that measured with a commercial hydrophilic PP blood filter (about 50% retention). Physical retention of leukocytes is only efficient if the membrane material is anti-biofouling, and so, does not interact with other blood components able to trigger leukocyte attachment/deformation. A. Venault, C.-C. YeH, N.-T. Hsieh and Y. Chang, 2019 This chapter presents current efforts to design smart materials for blood separation, which do not rely on molecular sieving only, but on mechanisms of interactions between the membrane and the blood component to isolate. Although concepts have been introduced, there is more to do than has ever been done on this topic. This chapter stresses the need for a specific combination of materials to separate the component of interest from the bloodstream without inducing blood coagulation. PEGylated, zwitterionic and pseudo-zwitterionic materials can all improve the hemocompatibility of the membrane design. But to perform a smart separation, a charge bias has to be introduced by incorporating charged polymers, or a stimuli-responsive polymer has to be grafted which interactions with the blood component are tuned by environmental conditions. Attention is also given to methods for preparing supporting layers, poly(vinylidene fluoride)-based or polypropylene-based. Finally, examples of reported smart blood separations are scrutinized, including the separation of proteins from whole blood, the development of leukocyte depletion or platelet concentration filters. We end this chapter with an identification of the current challenges to overcome to expand the development of smart membranes for blood separation. Technology
Zwitterionic is the future of biomedical materials
A brief summary of the Zwitterionic technology
The story of Zwitterionic technology
Zwitterionic technology
Zwitterionic-Bias technology: capable of capturing specify cell.
Leukocyte Filtration Mechanism
Publications
Developing blood leukocytes depletion membranes from the design of bio-inert PEGylated hydrogel interfaces with surface charge control
A zwitterionic interpenetrating network for improving the blood compatibility of polypropylene membranes applied to leukodepletion
Smart biomedical membranes for blood separation