普瑞博核心技術建構在創新的專利技術 – 表面雙離子化;它能廣泛應用於常見的材料上賦予表面生物惰性,使其相容於複雜的生物元素(例如人類血液)
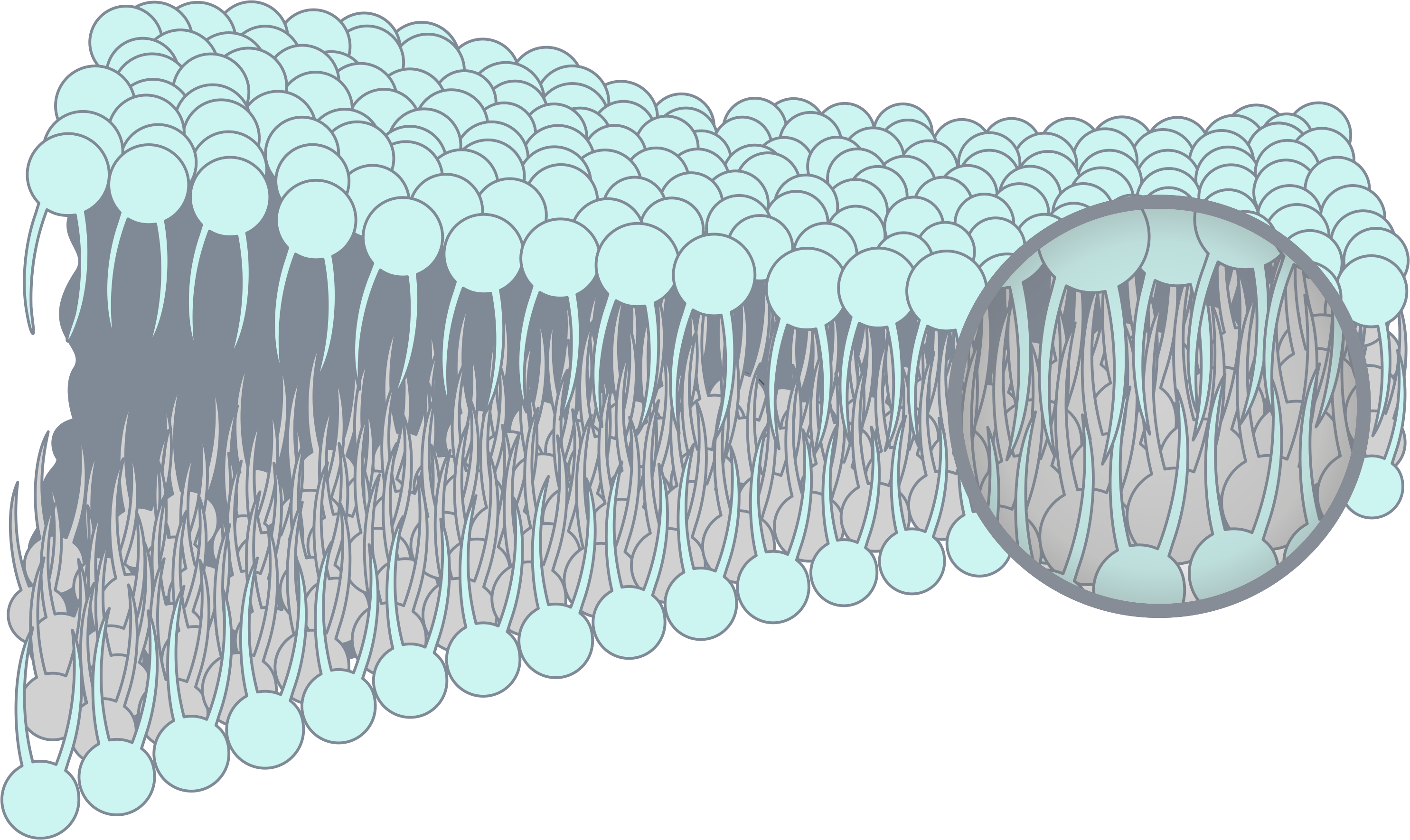
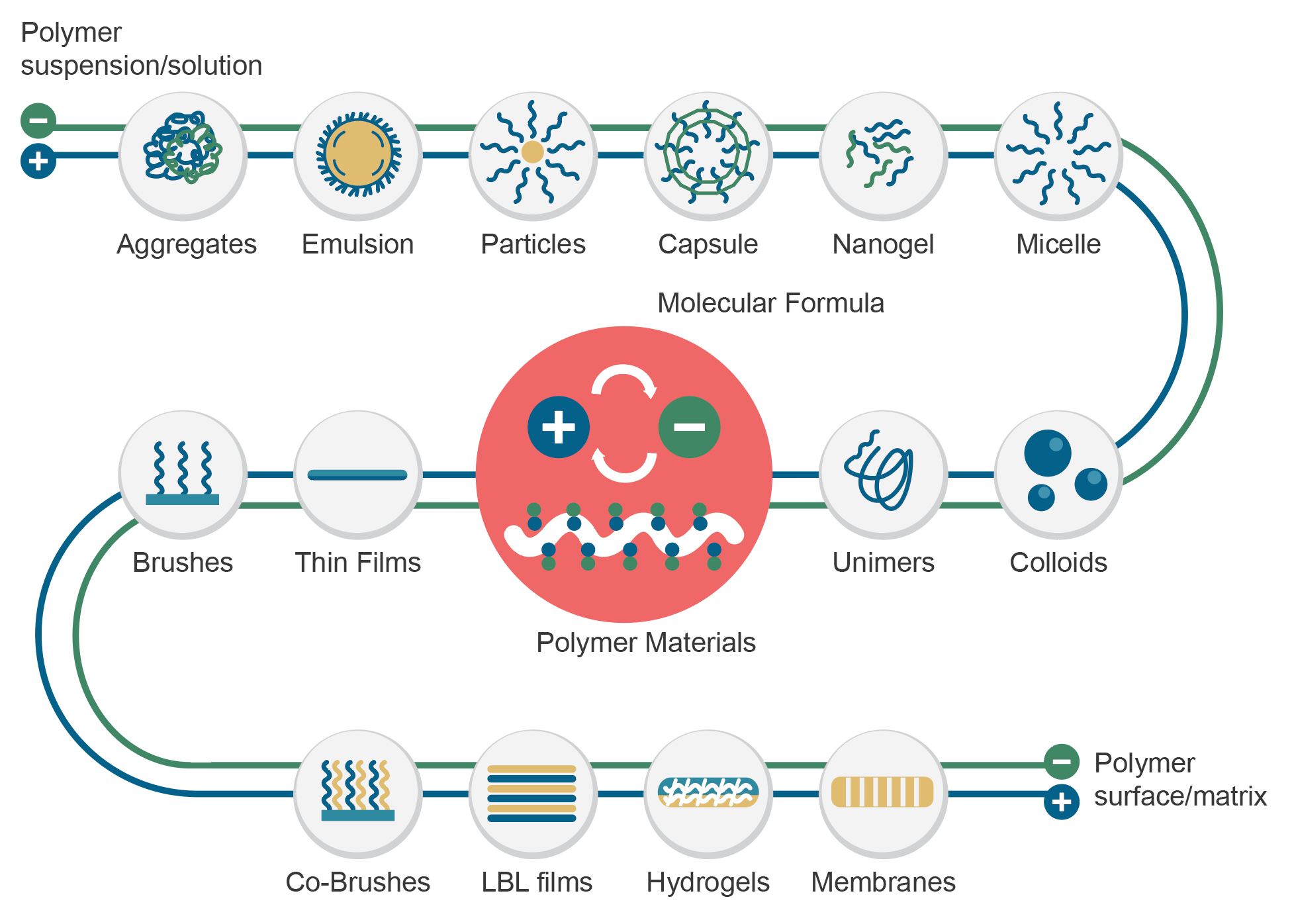
普瑞博的技術基礎為先進的抗沾黏技術,可廣泛應用於醫療器材甚至是日常用品之中,能在物品表面形成保水膜,使其具備高生物相容性及抗生物沾黏之特性。 在抗沾黏技術的基礎下,透過表面電性偏差設計,可以進一步賦予改質表面抓取特定細胞之特性,提供研究人員更為簡便的研究程序。 – Allan Hoffman 雙離子技術(後續簡稱ZWI-TEC)由張雍教授及其薄膜技術研發中心(RDCMT)的研究團隊於2006年展開研究,以期擴大在生物醫學應用的影響。 ZWI-TEC靈感取自細胞表面脂質雙層的排列結構。脂質層是細胞最外層屏障,能阻止蛋白質或其他物質擴散,同時防止有害物質進入。 由此,雙離子材料在所需材料表面形成保護層,能避免與生物物質接觸(沾黏),同時保有親水及高生物相容性等特性。 在ZWI-TEC的基礎上,研發團隊設計了雙離子的電荷偏差(即所謂的Zwitterionic-Bias),發現更多可能的應用。其中一項研究是從人類周邊血液中透過過濾方式捕捉幹細胞。在2009 年的這項研究中,張雍教授發現雙離子偏差膜表面上僅吸附白血球,並在接下來的6年反覆驗證,決定於2016年帶領團隊成立PuriBlood,將研究成果商業化,發展白血球減除過濾器。 雙離子聚合物的廣泛特性包括高親水性、低表面自由基、強水合力和弱生物分子作用力,對於常見的生物物質能產生抗黏附性。透過雙離子復合材料賦予一般類型的聚合物(如 PVC、PP、PE 或 EVA)上述特性,期待為下一代醫療器材開拓提供更安全、更方便的應用。 雙離子材料可形成以下聚合物型態: 雙離子偏差表面塗層技術(ZISC-bias technology)是新一代的生物智能材料,優於傳統HEMA、PEG等技術。 在雙離子膜層表面上,我們近一步設計其表面電性,透過控制正電荷和負電荷部分之間的平衡量,能專一吸附白血球,避免血漿蛋白質、血小板吸附於膜層,進而抑制血栓情形發生。 過濾膜層上層結構,ZISC-bias專利塗層擁有良好的血液相容性血液,可使血液快速平均分配及浸潤,並同時阻隔血塊及血液微聚體。(粗過濾) 在核心過濾層,ZISC-bias專利塗層賦予薄膜白血球捕捉專一性,並且讓紅血球或是其他血液成分能完整通過不被活化。(細過濾) Yan-Wen Chena, Antoine Venault, Jheng-Fong Jhong, Hsin-Tsung Ho, Chuan-Chuan Liu, Rong-Ho Lee, Ging-Ho Hsiue, Yung Chang, 2017 This study investigates the development of membranes for leukocytes depletion from model hydrogel interfaces. We first designed hydrogels from 2-(dimethylamino)ethyl methacrylate (DMAEMA) and poly(ethylene glycol) methyl ether methacrylate (PEGMA) to form poly(DMAEMA-co-PEGMA), and [2-(methacryloyloxy)ethyl] trimethylammonium chloride (TMA) and PEGMA to form poly(TMA-co-PEGMA). After characterizing the actual chemical composition of the hydrogels by XPS, efforts were made on studying the effect of the nature and content of amine functional group on the hydrophilicity and the surface charge of the gels. The adhesion of fibrinogen and blood cells was then methodically studied. Both types of gel are positively charged at pH 7.4, promoting fibrinogen and cell attachment. However, for the particular composition of 64% of DMAEMA in the hydrogel, results reveal that the adhesion of leukocytes was more important than that of erythrocytes and thrombocytes, thus suggesting that the control of the surface charge could lead to the selective adhesion of leukocytes. Based on these results, membranes were developed and tested in blood filtration for leukocyte depletion. Results prove that polypropylene membranes modified with a poly(DMAEMA-co-PEGMA) layer, containing 64% of DMAEMA can effectively lead to leukocyte depletion during blood filtration (99.6%) still maintaining a high erythrocytes recovery (98%). Cheng-Chi Lien, Po-Ju Chen, Antoine Venaulta, Shuo-Hsi Tang, Ying Fua, Gian Vincent Dizona, Pierre Aimar, Yung Chang, 2019 Although widely used in blood-contacting devices, polypropylene (PP) membranes are prone to biofouling by plasma proteins and blood cells. The present study explores the effect of a surface zwitterionization process on the improvement of the biofouling resistance of PP membranes for leukocyte reduction filters. The modification strategy consists in forming an interpenetrating network of poly(glycidyl methacrylate-co-sulfobetaine metha- crylate) (poly(GMA-co-SBMA) around the fibers of coated PP membranes, using a cross-linking agent: ethyle- nediamine (EDA). It is shown that with EDA, a range of poly(GMA-co-SBMA) concentration (1–5 mg/mL) leads to a 0°-water contact angle and high hydration of the networks without affecting the intrinsic porous structure of the material. Besides, the related membranes show excellent resistance to biofouling by Escherichia coli, fi- brinogen, leukocytes, erythrocytes, thrombocytes and cells from whole blood with reductions in adsorption of 97%, 86%, 90%, 95%, 97% and 91%, respectively, compared to unmodified PP. Used in whole blood filtration, it is demonstrated that in the best conditions (5 mg/mL copolymer, with EDA), leukocytes can be efficiently re- moved (> 99.99%) without altering the erythrocytes concentration in the permeate, and that leukodepletion is more efficient than that measured with a commercial hydrophilic PP blood filter (about 50% retention). Physical retention of leukocytes is only efficient if the membrane material is anti-biofouling, and so, does not interact with other blood components able to trigger leukocyte attachment/deformation. A. Venault, C.-C. YeH, N.-T. Hsieh and Y. Chang, 2019 This chapter presents current efforts to design smart materials for blood separation, which do not rely on molecular sieving only, but on mechanisms of interactions between the membrane and the blood component to isolate. Although concepts have been introduced, there is more to do than has ever been done on this topic. This chapter stresses the need for a specific combination of materials to separate the component of interest from the bloodstream without inducing blood coagulation. PEGylated, zwitterionic and pseudo-zwitterionic materials can all improve the hemocompatibility of the membrane design. But to perform a smart separation, a charge bias has to be introduced by incorporating charged polymers, or a stimuli-responsive polymer has to be grafted which interactions with the blood component are tuned by environmental conditions. Attention is also given to methods for preparing supporting layers, poly(vinylidene fluoride)-based or polypropylene-based. Finally, examples of reported smart blood separations are scrutinized, including the separation of proteins from whole blood, the development of leukocyte depletion or platelet concentration filters. We end this chapter with an identification of the current challenges to overcome to expand the development of smart membranes for blood separation. 核心技術
Zwitterionic is the future of biomedical materials
雙離子簡介
雙離子技術開發小故事
雙離子技術的應用
雙離子偏差技術:可抓取特定細胞
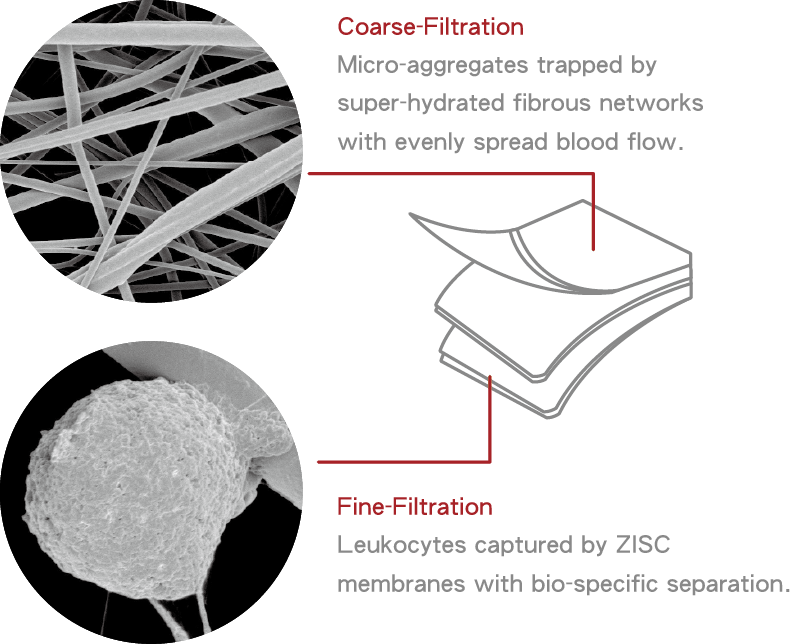
白血球減除過濾機制
期刊
Developing blood leukocytes depletion membranes from the design of bio-inert PEGylated hydrogel interfaces with surface charge control
A zwitterionic interpenetrating network for improving the blood compatibility of polypropylene membranes applied to leukodepletion
Smart biomedical membranes for blood separation