








Save Lives
Our Technology
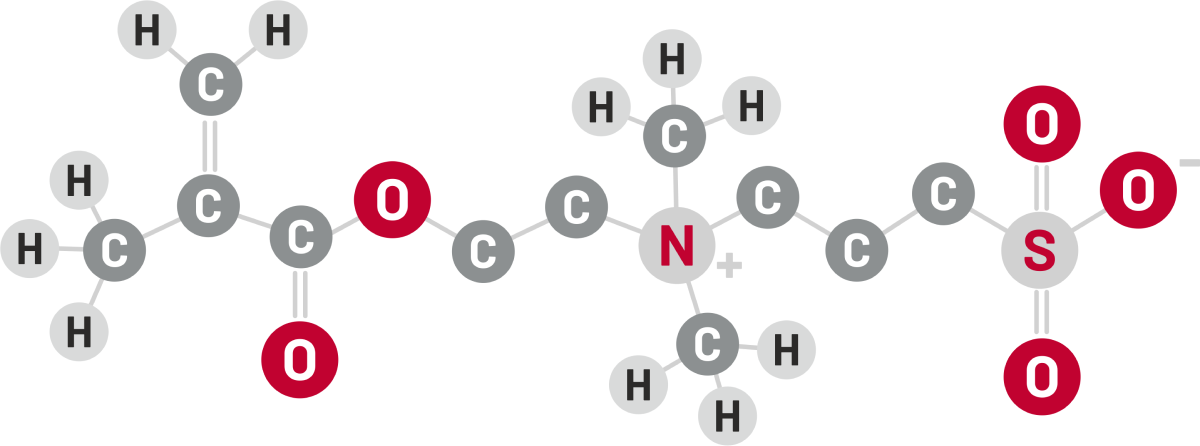
Discover biocompatible anti-fouling solutions with us in zwitterionic materials.
News









Discover biocompatible anti-fouling solutions with us in zwitterionic materials.

